Research Progress of Microglial BV-2 in Neurodegenerative Diseases
Neurodegenerative Diseases and Microglia
Neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s diseases, and so on, were referred to chronic and progressive loss of neurons in the brain and activation of microglia in brain cells[1]. The symptoms of neurodegenerative diseases include memory loss, impaired executive function, personality changes, and progressive inability to perform daily activities[2].
Microglia are immune cells in the central nervous system that mediate neuroinflammatory responses, which could perform immune monitoring, antigen presentation, and secretory regulatory immune molecules[3].
However, excessive activation of microglia can severely damage brain tissue and cause various neurodegenerative diseases such as Alzheimer’s disease (AD)[4]. As shown in figure 1, the microglia are activated in neurodegenerative diseases and express a large number of immune mediators such as IL-1β, TNFα, ROS, and NO. Microglial cells can be divided into two subtypes after activation: the M1-phased microglia and the M2-phased microglia. The M1-phased cells typically express pro-inflammatory cytokines, chemokines, and neurotoxic factors, while the M2-phased microglia generally produce anti-inflammatory, neuroprotective, and wound-healing factors[5, 6].
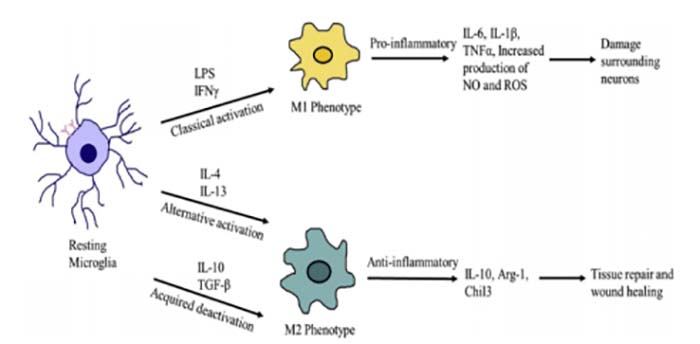
Figure 1. Depending on the type of stimuli, microglia can be activated and directed towards distinct phenotypes. [1]
Microglia plays a significant role in the research of neurodegenerative diseases, so further research on microglia may contribute to treat degenerative diseases.
BV-2 Mouse Microglia
BV-2 mouse microglia is an immortal cell line that was made by infecting with v-raf / myc recombinant retrovirus[7, 8]. BV-2 cells have the same functional phenotype as native primary microglia[9]. 90% of genes up-regulated in LPS-induced primary microglial cells, but it was less pronounced in BV-2 cells. Although BV-2 cells have a different inflammatory response from a primary culture, they are still valuable and widely used cell models of neuroinflammation[10].
BV-2 microglial activation and proliferation induce neuroinflammation and can cause various neurodegenerative diseases, including AD, PD, and multiple sclerosis. Many researchers use BV-2 cells to study the effects of drugs on neurodegenerative diseases[9, 11].
How to Culture BV-2 Cells
Many researchers have disputed the real-time cell morphology when subcultured because BV-2 is a semi-adherent and semi-suspended cell, which will directly affect the progress of subsequent related experiments.
Methods for some researchers to culture BV-2 cells: BV-2 cells were cultured in DMEM supplemented with 5% FBS (10% FBS) and 100 U/mL P-S before being maintained in a humidified incubator in a 5% CO2 atmosphere[5, 12]. This method currently has the highest success rate in culturing BV-2 cell, and the cell morphology is amoebic.
How to better culture BV-2 cells is very significant
BV-2 cells do not need to use trypsin during the passage, which could be blown down directly, and ameboid cell form was observed.
As shown in figure2, the cells were cultured for 96 hours in DMEM medium, supplemented with 10% FCS (BV-2FCShigh) or without FCS (BV-2øFCS). The difference in cell shape is clearly visible that the BV-2FCShigh cells retain curved-like round spots/ macrophage-like morphology; while the BV-2øFCS transforms into branch/spider-like morphology (visible in the window frame). In intermediate frequency images, tubulin (anti-mouse Ab bound to Alexa 488, green fluorescence) is used for cytoplasm, and DAPI (blue fluorescence) is used for nuclear staining [13].
The cells that were cultivated for a long term in the standard conditions with 10% of FCS (BV-2FCShigh) retained an ameboid morphology (Fig 2). Cell viability in both cultivation conditions was ≥ 95%. Therefore, 10% FBS should be used when culturing BV-2 cells.
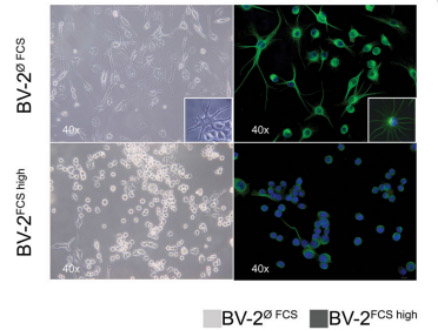
Figuer 2. Different cell morphology phenotype of the murine microglial BV-2 cell line has been shown with the bright field/phase-contrast (left panel) and immunofluorescence (IF) (right panel) images[13].
In recent years, a large number of papers reported that LPS induction substantially elevates the upsurge of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) via the MAPK signaling pathway in BV-2 glial cells[12]. Neuroinflammation leads to the activation of microglia and results in higher production of inflammatory markers. Many factors act on LPS-activated BV-2 cells to observe cell viability after administration[11, 14]. The activity of BV-2 cells was observed to clarify the effect of these drugs on neurodegenerative diseases and determine whether they may be a possible effective target for the treatment of degenerative diseases.
Measure the BV-2 Cell Viability
Briefly, BV-2 cells (1-5× 105 cells per well) were grown in 24-well plates which were incubated with LPS (1 mg/ml or100 ng/ml) for 30 min, then added different concentrations of the target reagent and co-culture with the cells for 24h, 48h, and 72h. You can use MTT, CCK8, and other methods to test the cell viability[5]. Meanwhile, the LPS-induced release of NO in the culture medium was determined. For example, to assess the effect of SLCN on the proliferation of BV-2 cells induced by LPS, Ganesan et al add LPS to BV-2 cells, then they added different concentrations of curcumin-loaded solid lipid nanoparticles SLCN and base curcumin, which induced the neuroinflammation [12]. They found that incubation with LPS alone significantly increased NO production in BV2 cells compared to controls. However, pretreatment with SLCN and basic curcumin can prevent this increase in NO production in LPS-stimulated BV2 cells (fig 3).
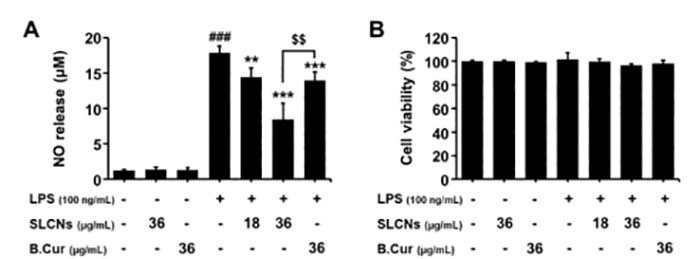
Figure 3. Effect of SLCN and base curcumin on NO production and cell viability in lipopolysaccharide LPS-stimulated BV2 cells[12].
Therefore, measuring the activity of BV-2 cells has a very critical role in researching the effects of different drugs on neurodegenerative diseases. The activity of BV-2 cells can reflect the degree of inflammatory damage in the nervous system and indirectly reveal the potential utility of drugs for neurodegenerative diseases.
Conclusion
BV-2 microglia were widely used in the research of extensive degenerative neurological diseases because characteristics are similar to the original microglia. Researchers who master the culture of BV-2 cells and some experimental methods to detect cell phenotype will better promote the research of neurological diseases.
AcceGen BV-2 Cell Line
AcceGen cultures and provides mouse BV2 microglia cell line, isolated from C57BL/6 mouse brain, transformed by recombinant retrovirus (v-raf/v-mic), for neurobiology research use.
For more detailed information, please visit our website or contact inquiry@accegen.com.
References
1. Subhramanyam CS, Wang C, Hu Q, Dheen ST: Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol 2019, 94:112-120.
2. Yeh FL, Hansen DV, Sheng M: TREM2, Microglia, and Neurodegenerative Diseases. Trends Mol Med 2017, 23(6):512-533.
3. Dwyer JB, Ross DA: Modern Microglia: Novel Targets in Psychiatric Neuroscience. Biol Psychiatry 2016, 80(7):e47-49.
4. Hwang JH, Wu SJ, Wu PL, Shih YY, Chan YC: Neuroprotective effect of tempeh against lipopolysaccharide-induced damage in BV-2 microglial cells. Nutr Neurosci 2019, 22(12):840-849.
5. Kim SM, Ha JS, Han AR, Cho S-W, Yang S-J: Effects of α-lipoic acid on LPS-induced neuroinflammation and NLRP3 inflammasome activation through the regulation of BV-2 microglial cells activation. BMB Reports 2019, 52(10):613-618.
6. Huang M, Li Y, Wu K, Yan W, Tian T, Wang Y, Yang H: Paraquat modulates microglia M1/M2 polarization via activation of TLR4-mediated NF-kappaB signaling pathway. Chem Biol Interact 2019, 310:108743.
7. E. Blasi RBVB, R. Mazzolla and F. Bistoni Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. Journal of Neuroimmunology, 1990, 229-237.
8. Yun HK, Park J, Chae U, Lee HS, Huh JW, Lee SR, Bae YC, Lee DS: Parkin in early stage LPS-stimulated BV-2 cells regulates pro-inflammatory response and mitochondrial quality via mitophagy. J Neuroimmunol 2019, 336:577044.
9. Wowro SJ, Tong G, Krech J, Rolfs N, Berger F, Schmitt KRL: Combined Cyclosporin A and Hypothermia Treatment Inhibits Activation of BV-2 Microglia but Induces an Inflammatory Response in an Ischemia/Reperfusion Hippocampal Slice Culture Model. Front Cell Neurosci 2019, 13:273.
10. Mendonca P, Taka E, Soliman KFA: Proteomic analysis of the effect of the polyphenol pentagalloyl glucose on proteins involved in neurodegenerative diseases in activated BV2 microglial cells. Mol Med Rep 2019, 20(2):1736-1746.
11. Wang Y, Zang W, Ji S, Cao J, Sun C: Three Polymethoxyflavones Purified from Ougan (Citrus reticulata Cv. Suavissima) Inhibited LPS-Induced NO Elevation in the Neuroglia BV-2 Cell Line via the JAK2/STAT3 Pathway. Nutrients 2019, 11(4).
12. Ganesan P, Kim B, Ramalaingam P, Karthivashan G, Revuri V, Park S, Kim JS, Ko YT, Choi DK: Antineuroinflammatory Activities and Neurotoxicological Assessment of Curcumin Loaded Solid Lipid Nanoparticles on LPS-Stimulated BV-2 Microglia Cell Models. Molecules 2019, 24(6).
13. Kucic N, Racki V, Jurdana K, Marcelic M, Grabusic K: Immunometabolic phenotype of BV-2 microglia cells upon murine cytomegalovirus infection. J Neurovirol 2019, 25(4):496-507.
14. Dingzhou Zhou1 YJ: Sirtuin 3 attenuates neuroinflammation-induced apoptosis in BV-2 microglia. AGING 2019, 11.
Comments
Post a Comment