Leukopak, an Ideal Choice for Large-scale Research or Pre-clinical Trials instead of PBMC
Human single cells are the key materials for research and production of cell therapy products. Yet, the purchase of fresh cells may be restricted, so there is an urgent need for suitable alternative cells that are viable and powerful[1]. Leukopak is the product of this demand.
What are Leukopaks and Leukapheresis?
Leukopaks (LPs) are leukapheresis samples obtained from healthy peripheral blood [2]. Leukapheresis is a specially modified apheresis technique designed to remove leukocyte and return the remaining cells (i.e, granulocytes, platelets, red blood cells) to the donor. The operation of plasma leukopenia can be performed in vitro with a small amount of liquid (200-300mL of plasma). It is not necessary to perform plasma exchange and blood product perfusion before surgery. Therefore, this is an ideal method for obtaining blood components from participants without exposing them or their blood to other people’s substances[3].
The percentage of cells can vary from donor to donor. As shown in figure1, flow cytometry analysis of LPs from 5 donors using a panel of recombinant fluorescently labeled REAfinity antibodies recognizing different surface antigens was performed. T cells, B cells, and monocytes account for approximately 55% (range 45-60%), 9% (5-15%), and 27% (range 10-30%), 3% granulocytes, and 3 % hematocrit[2].
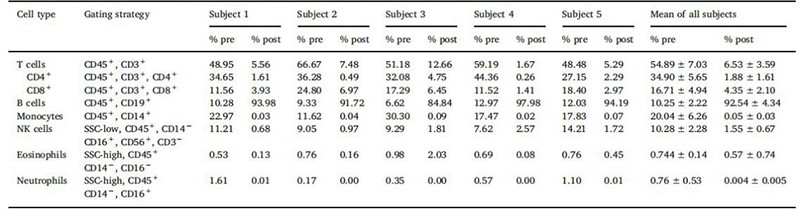
Figure1. Cell composition before and after cell isolation with Miltenyi StraightFrom® LP CD19 MicroBead Kit
Leukopak vs PBMC
MACS (magnetic-activated cell sorting)- and FACS (fluorescence-activated cell sorting)- purification methods require peripheral blood mononuclear cells (PBMC). Although preparing a small amount of PBMC was relatively simple, large-scale purification is challenging. This can be solved by using Leukopaks (LPs) from donor leukocytes, during which blood is unnecessarily separated into its constituent cells. When processing blood, each LP contains about 20 times more B cells than a single blood unit [2]. All LeukoPaks come from the adult healthy donors and one donor can collect up to 12 Leukopak collections in one year [4]. Therefore, Leukopaks (LPs) are rich in PBMCs, and their concentrations are much higher compared to those obtained by standard venipuncture collection methods or buffy coat products.
How to isolate the required cells from Leukopak?
Participants were evaluated during the pre-screening visit to ensure that all clinical safety indicators were met, and the donor criteria for the screening were scheduled for leukocyte puncture[5].
PBMCs isolation from Leukopak
As shown in figure2, once the leukocyte separation procedure was completed, the LeukoPaks (LPs) was marked with a unique participant identification number (PID #) and sent to the laboratory within one hour at room temperature. On average, the LP volume of 167 ml PBMC isolation process started within 2 hours. The PBMC isolation program draws on the density gradient method from the whole blood [4]. PBMCs were isolated from 5 ml heparinized venous blood of non-reactions and healthy donors by density gradient centrifugation at 500 g over Ficoll–Hypaque [6]. When the PBMC cell layer is transformed into a white lymphocyte layer, the cells were taken into a clean centrifuge tube by using a pipette for subsequent further separation of the target cells.

Figure2. Leukopak processing. The steps for processing a leukapheresis product correctly are shown in chronological order [4].
The Application of Leukopak in HIV diseases
The main obstacle to curing HIV-infected individuals is the existence of a reservoir of latently infected cells. These rare cells carry integrated HIV provirus but usually do not express viral proteins, making them invisible to the immune system and difficult to eliminate[7].
Leukopaks from healthy anonymous donors were used for primary T cell isolation. Cells were suspended at 5ⅹ107 cells/ml in PBS + 2% FBS and CD8+ cells were enriched by negative selection after a brief rinse in PBS + 2% FBS. The purified CD4 T cells were activated with 50 ng/ml PMA and 1 mM ionomycin for 72 hours. After 2 days of incubation with cCAR-T cells or matched untransduced CD8 T cells, cell-associated RNA was extracted, and HIV RNA was quantitated by droplet digital PCR. They observed a significant reduction in the amount of HIV RNA in cultures, including cCAR-T cells, but not in cultures including MicAbodies and untransduced parental CD8 T cells or unarmed CAR-T cells [8].

Figure3. Ex Vivo Killing of Reactivated CD4 T Cells from HIV-1-Infected Individuals ART by cCAR-T and MicAbodies[8].
Conclusion:
In the early days, whole blood was used to separate PBMC. According to general experience, up to 400cc of whole blood is collected, and such donors must have a long interval (such as 6 Months) to be collected again. The PBMC obtained by Leukopaks was 14 times more than the whole blood density gradient centrifugation process. Donors can repeatedly donate at 30-day intervals, which makes Leukopak an ideal choice for large-scale research or pre-clinical trials.
Where to buy Leukopak?
AcceGen provides Human Whole Leukopak and Various Diseased Leukopaks for pathology and pharmacy research. We hope our Leukopak products can accelerate your research process and make it more efficient at a lower cost.
AcceGen Leukopak Category:
| ABC-TC4376
| Human Whole Leukopak
| ABC-TC4293
| Human Fresh Systemic Lupus Erythematosus Leukopak
|
| ABC-TC4294
| Human Multiple Sclerosis Leukopak
| ABC-TC4296
| Human Crohn’s Disease Leukopak
|
| ABC-TC4297
| Human Hepatitis B Leukopak
| ABC-TC4299
| Human Hepatitis C Leukopak
|
| ABC-TC4301
| Human HIV-1 Leukopak
| ABC-TC4303
| Human HIV-2 Leukopak
|
| ABC-TC4305
| Human Osteoarthritis Leukopak
| ABC-TC4307
| Human Rheumatoid Arthritis Leukopak
|
| ABC-TC4309
| Human Psoriasis Leukopak
| ABC-TC4310
| Human Type 1 Diabetes Leukopak
|
| ABC-TC4311
| Human Type 2 Diabetes Leukopak
|
Reference
1. Wang W, Hexom T, Nucho L-M, Eastwood G: Cryopreservation of leukopaks show maintained viability and functionality. Cytotherapy 2015, 17(6):S22.
2. Ferrara F, Kolnik M, D’Angelo S, Erasmus FM, Vorholt D, Bradbury ARM: Rapid purification of billions of circulating CD19+ B cells directly from leukophoresis samples. N Biotechnol 2018, 46:14-21.
3. Ganzel C, Becker J, Mintz PD, Lazarus HM, Rowe JM: Hyperleukocytosis, leukostasis and leukapheresis: practice management. Blood Rev 2012, 26(3):117-122.
4. Garcia A, Keinonen S, Sanchez AM, Ferrari G, Denny TN, Moody MA: Leukopak PBMC sample processing for preparing quality control material to support proficiency testing programs. J Immunol Methods 2014, 409:99-106.
5. Roberts N, James S, Delaney M, Fitzmaurice C: The global need and availability of blood products: a modelling study. The Lancet Haematology 2019, 6(12):e606-e615.
6. Saini C, Tarique M, Ramesh V, Khanna N, Sharma A: gammadelta T cells are associated with inflammation and immunopathogenesis of leprosy reactions. Immunol Lett 2018, 200:55-65.
7. TAE-WOOK CHUN1 MARIA FISCHETTE2 DE, SOHEE PARK3 JULIA A. METCALF1 1 , STEPHANIE B. MIZELL1 , RICHARD T. DAVEY, JR. 1, JOANN M. MICAN1 H. CLIFFORD LANE1, CLAIRE W. HALLAHAN1 , MARK DYBUL1, M. MICHELLE BERREY4 & ANTHONY S. FAUCI1: Effect of interleukin-2 on the pool of latently infected,resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nature 1999.
8. Herzig E, Kim KC, Packard TA, Vardi N, Schwarzer R, Gramatica A, Deeks SG, Williams SR, Landgraf K, Killeen N et al: Attacking Latent HIV with convertibleCAR-T Cells, a Highly Adaptable Killing Platform. Cell 2019, 179(4):880-894 e810.
Comments
Post a Comment