Development and Application of CRISPR/cas9 Gene Editing Technology
This year, Emmanuelle Charpentier and Jennifer A. Doudna were awarded the Nobel Prize in Chemistry in recognition of their contributions to gene editing technology[1]. They developed one of the sharpest tools in gene technology: CRISPR-Cas9, which was known as “genetic scissors”. Scientists are able to precisely alter the DNA of animals, plants and microbes by using CRISPR/Cas9 technology[2].
The CRISPR-Cas9 system found in Streptococcus thermophilus uses this mechanism to resist invading viruses. In the past few years, this mechanism has brought revolutionary changes to DNA engineering and biomedical research[3].
The principles in gene editing
CRISPR consists of a small set of DNA sequences found in prokaryotic genomes that have been obtained from previous phage infections[4]. The CRISPR/Cas9 system consists of a targeting specific single guide RNA (sgRNA) and a Cas9 endonuclease. Targeting specific sgRNA which formed by the fusion of CRISPR RNA (crRNA) and transduction CRISPR RNA can guide Cas9 protein to the target site for cleavage, forming a double-strand break (DSB)[5]. Then, the DSB was repaired through non-homologous end joining (NHEJ)-mediated destruction or homology-directed repair (HDR)-mediated modification (shown in figure 1).
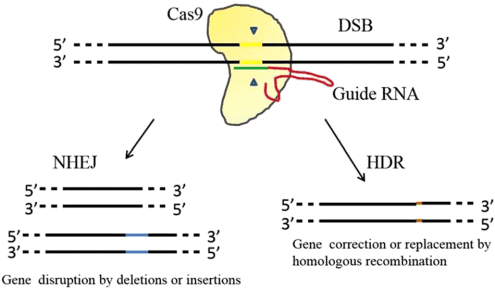
Figure 1. The clustered regularly interspaced short palindromic repeat (CRISPR)/ Cas9 system for targeted genome editing[6].
Many researchers constructed gene modified cell line or mouse by CRISPR-Cas9 to study the effects of target gene changes through knockin and knockout, etc.[7].
The applications of gene editing
CRISPR/Cas9 is used to research on cell proliferation, migration and invasion, and to imitatively repair disease-causing genes at the embryonic level. For example, Traf3 knockout HepG2 cell line was constructed by using gene editing technology in the CRISPR /Cas9 system to explore the role of Traf3 in hepatocarcinogenesis development. After construction, the cell lines were identified using PCR amplification electrophoresis and Western Blot technology. Then, the proliferation and invasion of the knockout cell lines were studied. The results showed that knockout of Traf3 gene by CRISPR/Cas9 system enhanced the proliferation, migration and invasion of HepG2 cells, providing an effective tool to study the function and mechanism of Traf3 [8]. This will make a significant contribution to the targeting of human disease diagnosis and treatment through the study of the effects and mechanisms of specific factors on cell growth and thus potentially on human disease.
In recent years, researchers want to correct disease-causing mutations in human embryos. Repairing disease-causing genes at the embryo level has the potential to reduce the burden of genetic disease and will likely improve fertility treatment for couples with disease-causing mutations.
Zuccaro et al. recruited a blind patient as a sperm donor and injected the sperm into oocytes along with a CRISPR editing tool. They derived two embryonic stem cell (ESC) lines with the expectation that the mutation will be repaired during embryonic cell development to produce a healthy embryo. However, as shown in figuer2, the researchers observed that half of the double-stranded DNA breaks were not repaired under CRISPR editing after analyzing the genomes of these early embryos, which resulted in a partial or even complete loss of chromosome 6 where the mutant EYS gene is located [9]. This also indicates that there are still significant challenges in editing human embryos using CRISPR technology.

Figure2. Visual summary of oocyte results
Conclusions
Up to date, the Crispr/Cas9 gene editing technology is stable and efficient, so it has been used by researchers all over the world in gene repair and genetic modification of various organisms. As for the people who are optimistic about science, it is easy to see the great commercial prospects of gene editing technology in biology, medicine, as well as agriculture and health. But behind the rational optimism, we cannot help but be wary of the enormous technical risks and ethical dilemmas that gene editing technology may bring to mankind. For example, the Chinese scientific researcher He Jiankui cut the genes of the twins to make them resistant to HIV infection after birth. The publicity of the trial immediately caused widespread concern.
Therefore, various countries should also formulate corresponding measures and legislation on the boundaries of the application of gene editing technology as soon as possible, in order to guard some of the forbidden areas of life science exploration for the foreseeable time.
How can AcceGen help you?
AcceGen is committed to offering a series of transfected stable cell lines like knockin, knockout, knockdown, and reporter cell lines developed by the CRISPR/Cas9 system. These cell lines provide you with a convenient means to study gene functions. Our experienced team can always come up with professional services tailored to your needs. Please feel free to contact us at 1-862-686-2696, or send an email to inquiry@accegen.com. Your questions and requests will be answered by expert staff in AcceGen within 24 hours.
References:
1. https://www.nobelprize.org/prizes/chemistry/2020/press-release/
2. Kiel T. Butterfield PSM, Chann Makara Han, Igor Kogut, and Ganna Bilousova: Generation of an Induced Pluripotent Stem Cell Line with the Constitutive EGFP Reporter; 2020.
3. Deshpande K, Vyas A, Balakrishnan A, Vyas D: Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 Genetic Engineering: Robotic Genetic Surgery. Am J Robot Surg 2015, 2(1):49-52.
4. Lander ES: The Heroes of CRISPR. Cell 2016, 164(1-2):18-28.
5. Yip BH: Recent Advances in CRISPR/Cas9 Delivery Strategies. Biomolecules 2020, 10(6).
6. Wu M, Hu N, Du X, Wei J: Application of CRISPR/Cas9 technology in sepsis research. Brief Funct Genomics 2020, 19(3):229-234.
7. Kherraf ZE, Conne B, Amiri-Yekta A, Kent MC, Coutton C, Escoffier J, Nef S, Arnoult C, Ray PF: Creation of knock out and knock in mice by CRISPR/Cas9 to validate candidate genes for human male infertility, interest, difficulties and feasibility. Mol Cell Endocrinol 2018, 468:70-80.
8. Hu W, Guo G, Chi Y, Li F: Construction of Traf3 knockout liver cancer cell line using CRISPR/Cas9 system. J Cell Biochem 2019, 120(9):14908-14915.
9. Zuccaro MV, Xu J, Mitchell C, Marin D, Zimmerman R, Rana B, Weinstein E, King RT, Palmerola KL, Smith ME et al: Allele-Specific Chromosome Removal after Cas9 Cleavage in Human Embryos. Cell 2020.
Comments
Post a Comment