Luciferase reporter genes: How to use them in molecular biology?
Bioluminescence is a powerful imaging modality for monitoring molecular and cellular features in real time [1]. Luciferases, known as bioluminescent enzymes, can catalyze the oxidation of small molecule substrates, such as luciferins, to release visible light. Currently, the luciferase reporter gene is a highly versatile tool for in vitro and in vivo assays and the corresponding reporter assay is extremely sensitive, reproducible. Therefore, luciferases have been employed as efficient reporters in a wide range of applications, including gene regulation and signaling, protein-protein interactions, drug screening, molecular imaging, cell-based assays, and noninvasive in vivo imaging [2-3].
Luciferases
Luciferases originates from luminescent organisms, such as terrestrial and marine organisms. And here luciferases are mainly classified into two categories: D-luciferin-dependent luciferases and coelenterazine-dependent luciferases.
D-luciferin-dependent luciferases
Firefly luciferase: probably the most popular as a reporter molecule due to the early discovery, high quantum yield of bioluminescence, availability of thermostable mutant variants with enhanced spectral characteristics and ease of production in bacteria. Firefly luciferase (Fluc) catalyzes the oxidation of D-luciferin (D-luc) in the presence of ATP, releasing primarily yellow-green light (Figure 1A). Fluc has been widely used in various in vitro and in vivo systems to assay metabolites involved in cell communication and cell signaling, to quantify protein–protein and protein-ligand interactions and to detect pathogenic bacteria and viruses.
Click beetle luciferases: the second most popular group of D-luc-dependent luciferases is isolated from the click beetle Pyrophorus plagiophthalamus. Engineered variants are available commercially.
Coelenterazine-dependent luciferases
Renilla luciferase: a medium-sized (36 kDa) cytosolic protein from a coral producing a steady luminescent signal. Early discovery and the availability of engineered versions with increased brightness and red-shifted spectra made this system more popular applications for biomedical research, particularly in drug-screening and bioimaging.
Gaussia luciferase: a small (20 kDa) secreted protein produced by a small crustacean from the group Copepoda, with high catalysis rate and exceptional thermostability. The signal of Gaussia luciferase scales linearly with the number of cells being assayed making this system useful for monitoring drug response and tumor progression. Both Renilla luciferase (RLuc) and Gaussia luciferase can catalyze coelenterazine, the bioluminescent substrate found in marine species, releasing blue light (Figure 1B).
Nanoluc luciferase: an engineered variant of a luciferase from the shrimp Oplophorus gracilirostris. The most striking NanoLuc can offer enhanced stability which has smaller size than Fluc and Rluc. This small (19 kDa) protein can catalyze a cell-permeable coelenterazine analogue, lacks disulfide bonds, to produce a bright signal suitable for a broad range of biological and bioapplications. Fusions with fluorescent proteins result in bright engineered bioluminescent constructs with red-shifted spectra facilitating single-cell and whole-body bioluminescent imaging in vivo. One of the drawbacks of this system is the high cost of reagents [4, 5].
At last luciferase reporters commonly used for bioluminescence imaging and their specifications are listed in Table 1 [6].
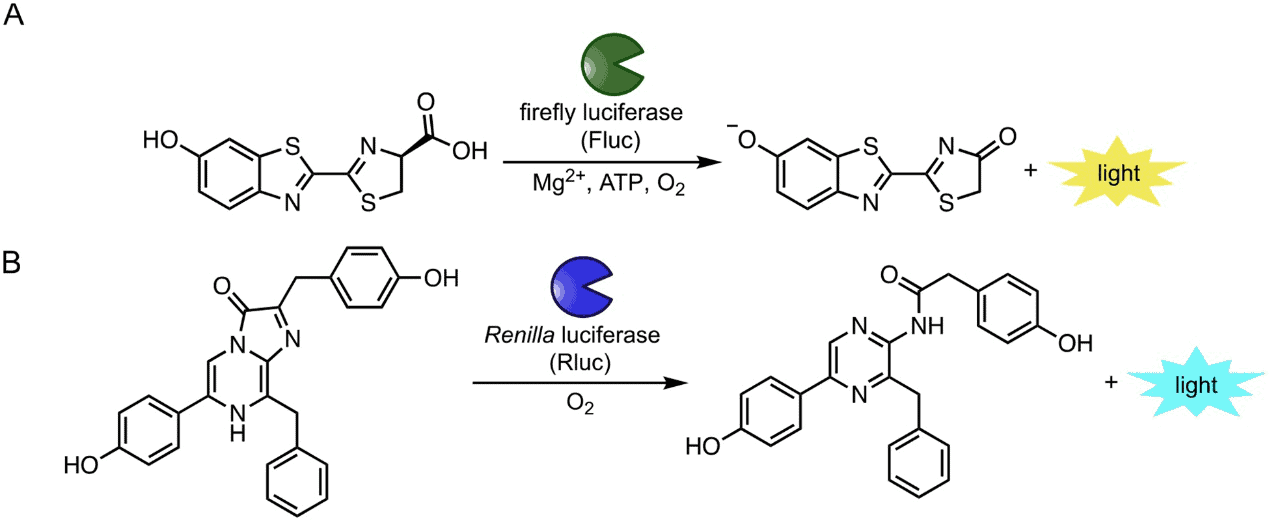
Figure 1: Popular bioluminescent enzymes and substrates. (A) Insect luciferases (including Fluc) catalyze the oxidation of D-luciferin (D-luc), resulting in photon production. (B) Marine luciferases (including Rluc) produce blue light via oxidation of coelenterazine substrates [4]

Table 1. Luciferase reporters commonly used for bioluminescence imaging and their specifications.
Luciferase-based gene reporter system
Luciferases are generally considered as reporter genes and their expression is easily and quantitatively detected. Therefore luciferases reporter genes can be attached to a regulatory sequence of a target gene for investigating its regulation or intracellular location. Figure 2 shows several examples of genetic constructs used in luciferase reporter gene technology.

Figure 2: Reporter gene constructs. Different configurations can be exploited by placing the reporter gene under control of a constitutive promoter (const. p), a promoter with tissue specific activation (tissue sp. P), or an inducible promoter (inducible p). Different combinations of promoters can be used to express two genes (target gene and reporter gene or two reporter genes) as a fusion protein or separated by internal ribosome entry site sequences [7].
Cells can be genetically modified by introducing a luciferase reporter gene fused to a regulatory DNA sequence. In general, using viral transduction, stable reporter cell lines expressing specific luciferase proteins are generated, and can be detected in a laboratory setting where bioluminescence imaging was used for tracking the luciferase signal.
Recent bioluminescence imaging applications
Bioluminescence imaging (BLI) is an optical molecular imaging technique used to visualize molecular and cellular processes in health and diseases and to follow the fate of cells with high sensitivity using luciferase-based gene report system. Recent applications for BLI are introduced in brief here.
Novel light-producing transgenic animals
Light-producing transgenic animals (LPTAs) have been designed for the in vivo monitoring of transcriptional activity, in which a constitutive or tissue-specific promoter drives the expression of luciferase. Also LPTAs have been recently developed for cancer research, metabolic research. Luminescent spontaneous mouse cancer models, such as MMTV-Luc2PyVT mice for mammary tumor development and EL1-Luc/Tag mice of acinar cell carcinomas, allow the longitudinal monitoring of tumor growth [8, 9]. And the above two models can also be employed to study drug response.
Transgenic zebrafish are also emerging animal models for stem cell and tissue regeneration research, based on high tissue regenerative capacity and low maintenance costs [10].
BLI of immune system
BLI has been used to image the immune cells, particularly in transplantation studies. At present the cellular and antibody-based immunotherapies attract increasing interests of researchers and then to elucidate the role of different immune cells in vivo has become very important for the design and use of these improved therapies. Transgenic mice models that allow multimodal imaging of T cells, NK cells, and DC cells are available [11-13].
BLI of extracellular vesicles
Extracellular Vesicles (EVs) are naturally secreted compartments and have important roles in cell-cell communication. BLI is ideally suited to elucidate the role of cell-cell communication in living systems. Gupta et al. have explored the bioluminescent labelling of EVs using different luciferase enzymes tethered to CD63 to achieve a highly sensitive system for in vitro and in vivo tracking of EVs and show that their distribution to different internal organs occurs just minutes after administration [14].
Perspectives
It is expected that more bioluminescent reporters such as luciferases will become available to facilitate biological discoveries. Moreover, with the assistance of gene-editing techniques, the applications of genetically encoded bioluminescent reporters will quickly expand by developing better bioluminescent cell lines and transgenic animals to fit diverse applications.
What can AcceGen do for you?
AcceGen proudly develops innovative technologies for life-science industry. AcceGen can easily engineer Reporter Stable Cell Lines for almost any applications such as screening of high-throughput RNAi, peptide, and chemical libraries, regardless of cell type with lentiviral-based transduction. We are committed to offering products in a large range of reporter cell lines (GFP / RFP / Luciferase) to help relative researches to move forward. For more detailed information, please visit please call us at 1-862-686-2696 or send an email to inquiry@accegen.com. Your questions and requests will be answered by our expert staff within 24 hours.
Reference
1. Miranda A. Paleya and Jennifer A Prescher: Bioluminescence: a versatile technique for imaging cellular and molecular features. Medchemcomm. 2014 (3): 255–267.
2. Hsien-Wei Yeh and Hui-Wang Ai: Development and Applications of Bioluminescent and Chemiluminescent Reporters and Biosensors. Annu Rev Anal Chem 2019;12 (1): 129–150.
3. Yongcun Yan, Pengfei Shi, Weiling Song, Sai Bi: Chemiluminescence and Bioluminescence Imaging for Biosensing and Therapy: In Vitro and In Vivo Perspectives. Theranostics. 2019; 9(14): 4047–4065.
4. Sierra J. Williams and Jennifer A Prescher: Building biological flashlights: Orthogonal luciferases and luciferins for in vivo imaging. Acc Chem Res. 2019 Nov 19; 52(11): 3039–3050.
5. Aubin Fleiss & Karen S. Sarkisyan: A brief review of bioluminescent systems. Current Genetics 2019(65), 877–882.
6. Laura Mezzanotte, Moniek van ‘t Root, Hacer Karatas, Elena A Goun, et al.: In Vivo Molecular Bioluminescence Imaging: New Tools and Applications. Trends Biotechnol
2017 35(7):640-652.
7. L Cevenini, MM Calabretta, D Calabria, A Roda, E Michelini: Luciferase Genes as Reporter Reactions: How to Use Them in Molecular Biology? Adv Biochem Eng Biotechnol 2016
(154):3-17.
8. Agnieszka M Zagozdzon, Patrick O’Leary, John J Callanan, John Crown, William M Gallagher, et al.: Generation of a new bioluminescent model for visualisation of mammary tumour development in transgenic mice. BMC Cancer. 2012; 12: 209
9 Ning Zhang, Scott Lyons, Ed Lim, Peter Lassota: A spontaneous acinar cell carcinoma model for monitoring progression of pancreatic lesions and response to treatment through noninvasive bioluminescence imaging. Clin. Cancer Res. 2009; 15: 4915-4924
10. Chen-Hui Chen, Ellen Durand, Jinhu Wang, Leonard I Zon, et al.: zebraflash transgenic lines for in vivo bioluminescence imaging of stem cells and regeneration in adult zebrafish. Development 2013 140(24):4988-97.
11. Manishkumar R Patel, Ya-Fang Chang, Ian Y Chen, Michael H Bachmann, et al.: Longitudinal, noninvasive imaging of T-cell effector function and proliferation in living subjects. Cancer Res. 2010; 70: 10141-10149.
12. Janelle A Olson, Robert Zeiser, Andreas Beilhack, Joshua J Goldman, et al. Tissue-specific homing and expansion of donor NK cells in allogeneic bone marrow transplantation. J. Immunol. 2009; 183: 3219-3228
13. Ho Won Lee, Seung Yun Yoon , Thoudam Debraj Singh , Yoon Ju Choi, et al.: Tracking of dendritic cell migration into lymph nodes using molecular imaging with sodium iodide symporter and enhanced firefly luciferase genes. Sci. Rep. 2015; 5: 9865
14. Dhanu Gupta, Xiuming Liang, Svetlana Pavlova, Oscar P.B Wiklander, et al.: Quantification of extracellular vesicles in vitro and in vivo using sensitive bioluminescence imaging. J Extracell Vesicles. 2020; 9(1): 1800222.
Comments
Post a Comment