History and Classification of Human Leukemia Cell Lines
Leukemia cell lines are important and widely used research tools. Their usefulness is mainly connected with their ability to provide an indefinite source of biological material for experimental purposes. Due to their high relevance for human disease, easy manipulation, and relative low costs, leukemia cell lines continue to represent vital in vitro model systems for a large range of ongoing investigations, especially basic leukemia research and drug discovery.
History of Human Leukemia cell lines
In 1963, the first continuous human hematopoietic cell lines were established from Nigerian patients with Burkitt’s lymphoma by Pulvertaft at the University of Ibadan in Nigeria. The cell line RAJI is the best known culture of this series [1].
In 1970, the K562 cell line, the first hematopoietic cell line derived from chronic myeloid leukemia (CML), was established from the pleural fluid of a patient with CML in blast crisis [2].
Furthermore, the availability of recombinant growth factors and conditioned media allowed, especially during the 1980s and 1990s, the stabilization of a number of hematopoietic cell lines that cover almost all steps of myeloid and lymphoid leukemia subset classification (except in cases of CML in chronic phase).
In 1991, stabilization and characterization of the NB4 cell line were reported and this in vitro model system was then used for studying acute promyelocytic leukemia.
Furtherly, Table 1 lists historical milestone in the establishment of three important hematopoietic cell lines and their contributions to milestone in cancer research and health care.
Table 1. Historical milestone in the establishment of three hematopoietic cell lines and their contributions to milestone in cancer research and health care [3, 4, 5]
| Cell Line | Year of Publication | Year of Establishment | Disease | Cell type | Investigator | Number of Publications in the Cancer Field | Benefit for Public Health Care |
| RAJI | 1964 | 1963 | Burkitt NHL | mature B-cell | Pulvertaft | 1557 | Definition of the mechanisms of infection by Epstein-Barr virus |
| K562 | 1973 | 1970 | CML-BC | erythroid | Lozzio et al. | 8001 | Development of treatment protocols for chronic myeloid leukemia |
| NB4 | 1991 | 1989 | AML-MS | myelocytic | Lanotte et al. | 1227 | Development of treatment protocols for acute promyelocytic leukemia |
The number of publications was derived through access to PubMed on 28 May 2019.
Classification of Human Leukemia cell lines
The most often used classification of human leukemia-derived cell lines is based on the physiological spectrum of the normal hematopoietic cell lineages:
First, lymphoid versus myeloid;
Secondly, T cell, B cell, NK cell versus myelocytic, monocytic, megakaryocytic and erythroid;
Thirdly, certain specific subtypes, such as B cell precursor, mature B cell, plasma cell for the B cell lineage and myeloblastic, promyelocytic, eosinophilic, basophilic for the myelocytic cell lineage (Table 2).
Table 2 Classification of leukemia cell lines [3]
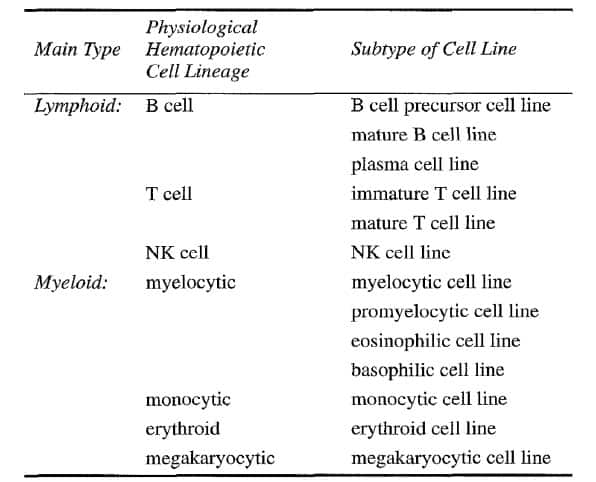
The most useful technique for assigning a given cell line to one of the major cell lineages is undoubtedly immunophenotyping. The more extensive and complete the immunopurified, the more precise is the categorization and classification of the cell lineage-derivation and status of arrested differentiation along this cell axis [3].
Indeed, the currently available cell lines include as follows: pre B-cell lines (101 model systems); B-cell lines (180 model systems); plasma cell lines (71 model systems); immature T-cell lines (59 model systems); mature T-cell lines (23 model systems); NK cell lines (11 model systems); myelocytic cell lines (77 model systems); monocytic cell lines (35 model systems); and, erythrocytic/megakaryocytic cell lines (49 model systems) [4].
Establishment and Culture of Human Leukemia Cell Lines
The history of leukemia research and the establishment of continuous leukimia cells are closely related. Specifically, methods of establishment and culture of human leukemia cells are as follows:
Acquisition of Cells
- 1.Collect heparinized or otherwise anticoagulated specimens of peripheral blood or bone marrow or other samples in sterile tubes. Place lymph nodes and other solid tissues in sterile containers.
- 2.Specimens should ideally be processed as soon as possible after receipt, but may be stored overnight at room temperature. Peripheral blood and bone marrow should remain undiluted, solid tissues should be placed in culture medium.
- 3.Cryopreserved samples can also be used for attempts to establish cell lines. But it appears to be of advantage to isolate the mononuclear cells prior to cryopreservation by Ficoll-Hypaque density gradient centrifugation
Isolation of Cells
- 1.Cut solid tissue specimens (e.g. lymph nodes) with scissors and force the particles through a fine metal mesh. Suspend the cells in 50-100 mL culture medium (possibly also more depending on the size of the tissue specimen). The most commonly used media are RPMI 1640, IMDM, MEM-a, or McCoy’s 5A.
- 2.Dilute the blood and bone marrow samples 1:2 with culture medium. Isolation of cells from a leukapheresis collection requires dilution of the sample with culture medium at 1:4.
- 3.Pipette the Ficoll-Hypaque density gradient solution (density 1.077 g/L) into a 15 or 30 mL conical centrifuge tube. Slowly layer the mixture of medium and sample over the Ficoll-Hypaque solution. Use equal volume of sample mixture and Ficoll-Hypaque solution.
- 4.Centrifuge for 20-30 min at 450 × g at room temperature (with the centrifuge brakes turned off). A layer of mononuclear cells should be visible on top of the Ficoll-Hypaque phase as they have a lower density than the Ficoll-Hypaque solution.
Culture Conditions
- 1.Adjust the cell suspension of the original patient cells to a concentration of 2-5 × 106 / mL in culture medium with 20% FBS plus additional 10% conditioned medium (CM) of cell line 5637 or with an appropriate concentration of purified or recombinant growth factors.
- 2.Place 5-10 mL of the cell suspension in the complete culture medium in an 80 cm2 plastic culture flask. If 24-well plates are used, add 1-2 mL cell suspension into each well. Add 100-200 mL of cell suspension into wells of 96-well flat-bottomed microplates.
- 3.Place the cells in a humidified incubator at 37°C and 5% CO2 in air.
- 4.Expand the cells by exchanging half of the spent culture volume with culture medium plus 20% FBS plus 10% 5637 CM (or with appropriate concentrations of recombinant growth factors) once a week. After a few hours, some cells become adherent.
- 5.During the first weeks, the neoplastic cells may appear to proliferate actively. If the medium becomes acidic quickly (yellow in the case of RPMI 1640 medium), change half of the volume of medium at 2-3 days interval (seldom daily). If the number of the cells increases rapidly, readjust the cells weekly to a concentration of at least 1 × 106 /mL in fresh complete medium by dilution or subdivision into new flasks or wells of the plate. The neoplastic cells from the majority of patients undergo as many as four doublings in 2 weeks, but after 2-3 weeks most malignant cells cease proliferating. Following a lag time of 2-4 weeks (crisis period), a small percentage of cells from the total population may still proliferate actively and may continue to grow forming a cell line.
- 6.If the malignant cells continue to proliferate for more than 2 months, there is a high possibility of generating a new cell line. Then, the task of characterizing the proliferating cells should be begun as soon as possible. Prior to the characterization of the cells, freeze ampoules of the proliferating cells containing a minimum of 3 × 106 cells/ampoule in liquid nitrogen to avoid loss of the cells due to occasional contaminations or other accidents.
- 7.Limiting dilution of the cells in 96-well plates leads to the generation of monoclonal cell lines [6,7].
Applications of Human Leukemia Cell Lines
How well do in vitro cell line models recapitulate the biologic processes of in vivo disease and drug response? Great effort has been taken by some research groups. The researchers evaluated most of the known hematopoietic cell lines regarding their immunological, cytogenetic, molecular, and functional features to evaluate that model system was able to best represent in vitro the disease from which it was derived. In this way, hematopoietic cell lines have been evaluated for the study of chronic myeloid leukemia; acute promyelocytic leukemia; human B-cell precursor leukemia; natural killer (NK) cell leukemia; acute leukemia with MLL gene alterations; acute erythroid leukemia. A large number of hematopoietic cell lines have been authenticated and characterized, and this amount encompasses nearly the whole spectrum of hematopoietic cell lineages and the various stages of differentiation along the respective cellular lineage [4].
Important milestones have been reached due to the correct use of hematopoietic cell lines in the hemato-oncological field, such as:
(1) the isolation of EBV, HIV, HTLV-I, and HHV-8 using the RAJI, HuT78/H9, CTCL2, and BC1 cell lines, respectively;
(2) the cloning of chromosomal translocations and the identification of relative fusion genes t (8; 14) MYC-IGH, t (9; 22) BCR-ABL, t (2; 5) NPM-ALK, t (1; 19) E2A-PBX1, t (4; 11) MLL-AF4, inv (16) CBFB-MYH11, t (8; 21) AML1-ETO, t (15; 17) PML-RARA, and t (14; 18) IGH-BCL2 using the RAJI, K-562, SU-DHL-1, 697, RS4;11, ME-1, Kasumi-1, NB4, and DoHH2 cell lines, respectively [33].
Notably, the NB4 cell line and the K562 cell line, these two important leukemia models, have made it possible to develop new therapeutic molecules that specifically target leukemic cells, similarly as in the cases of human acute promyelocytic leukemia and human chronic myeloid leukemia. In the first case, the NB4 cell line has been fundamental for the comprehension of the action of retinoic acid on the fusion gene PML-RAR alpha that was carried by malignant promyelocytic cells and for the development of diagnostic assays based on the PML pattern of distribution. In the second case, regarding the study of the BCR-ABL fusion protein (derived from t (9; 22)), it is important to highlight the role of the K562 cell line [4].
Conclusions
The advent of human leukemia-derived cell lines as a rich resource of abundant, accessible and manipulable living cells has contributed significantly to a better understanding of the pathophysiology of hematopoietic tumors [8]. And human leukemic cell lines remain essential tools for translating data obtained from sequencing experiments into novel therapies or diagnostic tests for leukemia. Furthermore, the selection of the most appropriate in vitro model system, followed by the selection of the culture conditions to best mimic the microenvironment in vivo, will influence the quality and reproducibility of the downstream experiments.
Where to Get Human Leukemia Cell Lines?
Human leukemia cell lines are ideal models for basic leukemia research and drug discovery. AcceGen provides the most authentic human leukemia cell lines for any specific research needs. There are various leukemia cell lines offered by AcceGen, such as acute promyelocytic leukemia (NB-4), acute monocytic leukemia (MONO-MAC-6), natural killer cell leukemia (KHYG-1) and so on.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact inquiry@accegen.com.
Reference
[1] Hans G DREXLER and Roderick AF MACLEOD: History of leukemia– lymphoma cell lines. Human Cell, 2010(23): 75-82.
[2] H. P. Koeffler and D. W. Golde: Human Myeloid Leukemia Cell Lines: A Review. Blood, 1980 (56): 344-350.
[3] Hans G. Drexler,Jun Minowada: History and classification of human leukemia– lymphoma cell lines. Leukemia & Lymphoma, 1998 (31): 305-316
[4] Peppino Mirabelli, Luigi Coppola and Marco Salvatore: Cancer Cell Lines Are Useful Model Systems for Medical Research. Cancers, 2019 (11): 1098-1115.
[5] Drexler, Hans G.: The Leukemia-Lymphoma Cell Line Factsbook, Elsevier Science & Technology, 2000.
[6] John M. Walker: Cancer Cell Culture: Methods and Protocols. Humana press, 2011.
[7] R. Ian Freshney, and Roswitha Pfragner: Culture of Human Tumor Cells. John Wiley & Sons, Incorporated, 2003.
[8] Hans G. Drexler, Yoshinobu Matsuo, Roderick A.F, et al: Continuous hematopoietic cell lines as model systems for leukemia–lymphoma research. Leukemia Research, 2000 (24) : 881 – 911.
Comments
Post a Comment