Human Myeloid Leukemia Cell Lines and Their Applications
Human myeloid leukemia cell lines provide useful model systems to study the control of differentiation in human myelogenous leukemia and, in a broader framework, the controls of normal myeloid development. Investigations with human myeloid leukemia cell lines should provide important insights into the cell biology and perhaps therapy of human leukemia. In present paper, HL-60 cell line and NB4 cell line are mainly introduced.
HL-60 cell line
Collins and coworkers established a human myelogenous cell line designated HL-60 from the peripheral blood of a female Caucasian patient with acute promyelocytic leukemia [1].The HL-60 cell line provides a unique in vitro model system for studying the cellular and molecular events involved in the proliferation and differentiation of normal and leukemic cells of the granulocyte/monocyte/macrophage lineage. A big advantage of HL-60 cells is that they are permissive to a range of genetic editing techniques, including lentiviral transduction, lipofectamine transfection, electroporation and nucleofection.
HL-60 cells were cultured initially in conditioned medium obtained from human embryonic lung cells, although later passages no longer required this medium for continued growth. At the morphological level, these cells appear to be in the promyelocyte stage and contain prominent azurophilic granules using a Romanowsky staining (Fig.1) [2].
HL-60 promyelocytic leukemia cells continuously proliferate in suspension culture with a doubling time of about 36 to 48 hours. This characteristic alone makes HL-60 unusual among human myeloid leukemias. Most fresh myeloid leukemia cells when cultured in liquid suspension undergo a limited number of cell divisions prior to growth arrest and cell death, and relatively few human leukemic cell lines displaying distinct myeloid or myelomonocytic characteristics have been established. HL-60 cell surface expression of transferrin and insulin receptors appears critical to their proliferative capacity. HL-60 cells can proliferate in serum free nutrient media(e.g., RPMI 1640) provided that it is supplemented with transferrin and insulin. The requirement for insulin and transferrin is absolute, as HL-60 proliferation immediately ceases if either of these compounds is removed from the serum-free culture media.
In addition, the characteristic of HL-60 cells that has attracted the most research interest is their capacity to differentiate in vitro to a variety of different cell types of the myelomonocytic lineage [3].Importantly, HL-60 cells can be differentiated into neutrophil-like cells by treating them with all-trans retinoic acid (ATRA), polar-planar compounds (eg, dimethyl sulfoxide [DMSO] and dimethylformamide [DMF]), actinomycin D or dibutyryl cyclic AMP (dbcAMP). Other compounds (e.g., vitamin D) may lead to differentiation towards a more monocytic phenotype [4]. Therefore, HL-60 cell line has become the most commonly used cell line in neutrophil research.
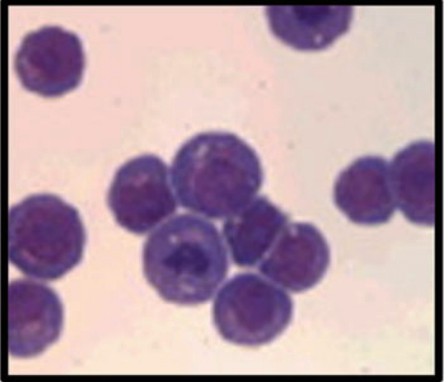
Fig. 1 HL-60 cells with Romanowsky stain
NB4 Cell Line
NB4 is a cell line originally established in 1991 from a female patient with relapsed acute promyelocytic leukemia. It is cultured in RPMI-1640 medium with serum supplementation, but without any other additives. The cell line has mainly attracted attention as a model to study cell differentiation in leukemia, but NB4 cells treated with ATRA display many features of terminally differentiated neutrophils and are therefore perfectly suitable for studying neutrophil function as well. In contrast to HL-60 cells, NB4 cells express a fusion protein that is formed between the retinoid receptor RARα and the tumor-suppressor protein promyelocytic leukemia (PML). This fusion protein, PML-RARα, binds to transcriptional targets of ATRA but does not initiate transcription. Addition of substantial amounts of ATRA displaces the fusion protein from the DNA and allows normal transcription of ATRA target genes. Differentiated NB4 cells are capable of producing ROS and possess functional azurophilic granules but do not contain specific or gelatinase granules; while the presence of both lactoferrin and MMP-9 has been demonstrated, they are not contained in granules. A study by Barber et al compared the changes in expression of CD markers between NB4 and HL-60 cells upon ATRA differentiation. The authors suggest that the difference in marker expression can be explained by the actions of PML-RARα in NB4 cells, whose function may differ from wildtype RARα even after ATRA binding [4].
General features of myeloid leukemia cell lines
For general features of myeloid leukemia cell lines, the features of leukemia-lymphoma (LL)-derived cell lines have been summed up properly and thus can be used for reference here because myeloid leukemia cell lines are one of main cell types of LL cell lines.
1. Malignant hematopoietic cell lines
The major advantage of leukemia cell lines is the unlimited supply of cellular material (Table 1). Furthermore, cell lines can be stored in liquid nitrogen and recovered without any detrimental loss of cellular features or cell viability [5,6].
Table 1
Major advantages of LL cell lines
| Unlimited supply of cell material |
| Worldwide availability of identical cell material |
| Indefinite storability in liquid nitrogen and recoverability |
2. Common characteristics ofLL cell lines
The following common characteristics of leukemia cell lines may be discerned in Table 2.
(1) The cell lines are monoclonal, i.e. presumed to be derived from one malignant precursor cell; however, subclones with variant features may emerge during extended culture.
(2) Their differentiation is arrested at a discrete stage during maturation in each cell lineage.
(3) Commonly, there is sustained, autonomous and external growth factor- independent proliferation of the cultured cells; this does, of course, not apply to new types of cell lines that were first developed in the late 1980s and that were deliberately established as constitutively growth factor-dependent cell lines.
(4) They contain genetic alterations: a survey of LL cell lines reported in the literature showed that among 429 well-characterized cell lines (excluding sister cell lines, subclones, EBVB-LCLs, Burkitt’s lymphoma- and ATLL-derived cell lines) for which karyotypes have been published, only two (0.5%) showed a normal karyotype without any structural or numerical aberrations. Besides these gross alterations at the cytogenetic level, many cell lines also carry alterations that are detectable only at the molecular level, e.g. point mutations or deletions of oncogenes, tumor suppress or genes, or other genes These genetic changes presumably provide the affected cell with either proliferative or survival advantages, and may play an important role in both the in vivo tumorigenesis and the in vitro establishment of the cell line.
(5) Finally, under optimal culture conditions, the salient features(including the classical and the molecular cytogenetic aberrations) will remain stable in long-term culture. However, deliberate experimental manipulations and intentional or accidental suboptimal culture conditions(e.g. contamination with mycoplasma, use of inappropriate culture media or supplements, over-dilution leading to ‘bottle-necking’ selections, etc.), with the ensuing stress on cells, may exert selection pressures that, in turn, result in phenotypic and genotypic shifts [5,6].
Table 2
Common characteristics of LL cell lines
| Monoclonal origin |
| Differentiation arrest at a discrete maturation stage |
| Sustained proliferation in culture Genetic alterations |
| Stability of most features in long-term culture |
Pitfalls in the use of cell lines: cross-contamination and mycoplasma infection
The exciting studies that were performed corroborate the importance and usefulness in all fields of medical research of cancer cell lines, with particular reference to cancer research and drug discovery. However, large percentages of LL cell lines are contaminated with mycoplasma (about 30%) or are cross-contaminated with other cell lines (about 15-20%) [5]. Then, it is important to highlight two outstanding problems: cross-contamination and mycoplasma infection.
The main reasons for both cross-contamination and mycoplasma infection are the same, namely, faulty cell culture techniques, inappropriate handling of cell lines, and a lack of knowledge and information regarding the consequences and effects of contaminants. Solutions to these problems are sensitive detection, effective elimination and rigorous prevention of mycoplasma infection, and proper, regular authentication of cell lines [5]. Undoubtedly, according to current guidelines, it is good practice to obtain a cell line of interest from a qualified source, such as international cell line banks. These infrastructures assure a full molecular and cellular characterization, which indicate the potential fields of application of each cell line, and routine tests areapplied to prevent cross-contamination as well as mycoplasma infection. In this way, these referenced cell lines assure improved research comparability, both geographically and with time.
Currently, the major cell line repositories include (1) the American Type Culture Collection (ATCC) (USA); (2) theLeibniz-Institute DSMZ; (3) the European Collection of Authenticated Cell Cultures (ECACC); (4)the Japanese Cancer Research Resources Bank (JCRB); the RIKEN BioResource Center (Japan); and,(5) the Korean Cell Line Bank (KCLB). In addition to these providers, we believe that institutional biobanks could play a critical role in preventing the occurrence of cross-contamination and mycoplasma infection.
Indeed, the centralized management of cell lines by qualified and experienced biobank personnel could be helpful for (1) distributing cell lines received from the accredited international repositories (ATCC, DSMZ, etc.); (2) generating a master (biobank staff only) and a working (biobank and institutional researchers) cell bank with different batches for each model system; (3) culturing each cell line according to the provided culture conditions; (4) routinely checking each biobanked modelsystem for cross-contamination and mycoplasma infection; and, (5) disseminating the importance of following the published guidelines when working with cell cultures [7].
Applications of Human Myeloid Leukemia Cell Lines
Using HL-60 and NB4 Modelling Acute Myeloid Leukemia
Eirin Alme et al. have designed and synthesized silver-3-alkyl-1-mesityl-1H- imidazol-3-ium complexes. These complexes were tested in vitro against the human cell lines HL-60, which models acute myeloid leukemia. Moreover, they also wanted to further optimize the cytotoxicity towards a potent compound where only a very small doseis required and thus fit into an appropriate therapeutic window. At last results shown that the complexes were found to be cytotoxicin the micromolar concentration range (13–4μM)towards HL-60 [8].
Paclitaxel (Ptx) combined with tanshinone IIA (TanIIA) was found to show synergistic effect on inducing apoptosis of human acute promyelocytic leukemia (APL) cell line NB4. To enhance the efficacy and reduce side effects, an active targeting drug delivery system with mesoporous silica nanoparticles (MSNs) coated with folic acid (FA) modified PEGylated lipid-bilayer (LB) membrane (FA-LB-MSNs) was established for co-loading drugs. According to Zhe Li et al., their results demonstrated that Ptx and TanIIA had a strong synergistic effect on NB4cells. FA-LBMSNs had good drug loading and tumor-targeted drug delivery property. Co-delivery of Ptx and TanIIA with FA-LB-MSNs for the treatment of APL could effectively be uptaken by NB4cells and promote apoptosis and differentiation of NB4cells, showing a strong tumor inhibition effect. The designed active tumor-targeting drug co-delivery systems provided method promising approach for the effective anti-tumor effect of chemotherapy [9].
HL-60 Cell Line as a Model for Human Neutrophils
The HL-60 cell line is most commonly used as a model for human neutrophil research. Compounds such as ATRA and DMSO are widely used to differentiate HL-60 cells into granulocytes. The complete overview of functions executed by differentiated HL-60 cells is summarized in Table 3. HL-60 Cell Line can be used to study chemotaxis, phagocytosis, respiratory burst in neutrophils, neutrophil extracellular traps and immunosuppressive function of neutrophils and so on. For example, as for studying chemotaxis in neutrophils (chemoattractant receptors on differentiated HL-60 Cells), HL-60 cells, upon granulocytic differentiation, have been shown to upregulate receptors for fMLF, LTB4, C5a, C3a, platelet-activating factor (PAF) and CXC chemokines).The levels of CXCR1 and CXCR2 have been reported to be relatively low compared to wild-type neutrophils. Interestingly, a study by Jacobs et al showed that the G-protein/receptor complexes for fMLF, LTB4 and C5a on DMSO-HL-60 have a different affinity for GTP binding, resulting in different potency of the chemoattractant. The authors demonstrate that DMSO-HL-60 cells preferentially respond to fMLF, less readily to C5a and least readily to LTB4 [4].

Table 3 Functions Which Differentiated HL-60 Cells are Capable of Executing
NB4 Application in Finding Rapid Diagnostic Method of APL
The aptamers with high affinity and specificity binding to acute promyelocytic leukemia (APL) NB4 cell line were selected from a random single-stranded DNA (ssDNA) library of systematic evolution of ligands by exponential enrichment (CELL-SELEX). The binding rate of FITC-ssDNA library and NB4 cells was monitored using flow cytometry and fluorescence microscope. Zhang Xian-hui et al. founded that the aptamer CX9 had the highest affinity, and the equilibrium dissociation constant (Kd) was 16.2 nM. And the fluorescence intensity of CX9 binding to NB4 cells was stronger than HL60 and K562 cells under fluorescence microscopy. Their study indicates that aptamer CX9 exhibits high affinity and specificity with NB4 cells and lay a foundation for the rapid diagnostic method to detect APL with fluorescence-labeled aptamer [10].
Conclusions
Human myeloid leukemia cell lines may provide the framework for studies leading to a deeper understanding of the control of differentiation inhuman myeloid leukemia and, in a broader perspective, the control of normal cellular differentiation. Future studies with these cell lines should shed new light on the cell biology of human myeloid leukemia and perhaps lead to new therapeutic modalities and rapid diagnostic method.
Where to get Human Myeloid Leukemia Cell Lines?
AcceGen provides various types of Human Myeloid Leukemia Cell Lines to meet all your research needs. HL60 and NB4 are AcceGen featured myeloid leukemia cell line products that backed by AcceGen advanced technology. More other types of leukemia cell lines are available in AcceGen: Human Leukemia Cell Lines
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact inquiry@accegen.com.
Reference
[1] H. P. Koeffler and D. W. Golde: Human Myeloid Leukemia Cell Lines: A Review. Blood, 1980(3) : 344-350.
[2] Paul C. Guest: Pre-Clinical Models: Techniques and Protocols. Humana Press, 2019: 239-240.
[3] StevenJ. Collins: The HL-60 Promyelocytic Leukemia Cell Line: Proliferation, Differentiation, and Cellular Oncogene Expression. Blood, 1987(5):1233-1244.
[4] Marfa Blanter, Mieke Gouwy, Sofie Struyf: Studying Neutrophil Function in vitro: Cell Models and Environmental Factors. Journal of Inflammation Research, 2021 :14 141–162
[5] Hans G. Drexler, Yoshinobu Matsuo, Roderick A.F. MacLeod: Continuous hematopoietic cell lines as model systems for leukemia– lymphoma research. Leukemia Research, 2000(24): 881 – 911.
[6] Hans G DREXLER and Roderick AF MACLEOD: History of leukemia-lymphoma cell lines. Human Cell, 2010(23): 75–82.
[7] Peppino Mirabelli, Luigi Coppola and Marco Salvatore: Cancer Cell Lines Are Useful Model Systems for Medical Research.Cancers, 2019(11): 1098.
[8] Eirin Alme, Karl Wilhelm Törnroos, Bjørn Tore Gjertsen, et al.: Synthesis of N-Aryl- and N-alkyl-Substituted Imidazolium Silver Complexes: Cytotoxic Screening by Using Human Cell Lines Modelling Acute Myeloid Leukaemia. Chem Med Chem., 2020(15): 1509–1514.
[9] Zhe Li, Yongtai Zhang, Chunyun Zhu, et al.: Folic acid modified lipid-bilayer coated mesoporous silica nanoparticles coloading paclitaxel and tanshinone IIA for the treatment of acute
promyelocytic leukemia. International Journal of Pharmaceutics, 2020( 586): 119576-119586.
[10]Xian-hui Zhang, Wei Wang, Xin Chen: Selection and identification of an ssDNA aptamer to NB4 cell. J Clin Lab Anal., 2021(35):e23718.
Comments
Post a Comment