Regulators of gene expression: MicroRNAs
- Get link
- X
- Other Apps
Non-coding RNA and Micro RNA
What is Non-coding RNA?
Non-coding RNAs (ncRNAs) refer to the RNAs that do not translate into proteins. There are many kinds of ncRNAs, and some of them play key roles in the physiological and pathological processes, such as transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), multiple small RNAs (such as microRNAs, siRNAs, snRNAs, etc.), and some long non-coding RNAs[1, 2]. Many ncRNAs have not yet been discovered, and there are also many ncRNAs whose functions have not been determined or are useless[3, 4]. The first ncRNA to be characterized was alanine tRNA in 1965s [5], and the structure of tRNA was resolved in the 1970s (Figure.1a) [6, 7]. And then as more ncRNAs were discovered, the importance of ncRNAs was determined (Figure.1b). NcRNAs are involved in the pathological process of various diseases, including cancer[8], autism[9], Alzheimer’s disease[10], etc. Therefore, it is considered to be a potential target for the treatment of many diseases and thus has become one of the current research hotspots.
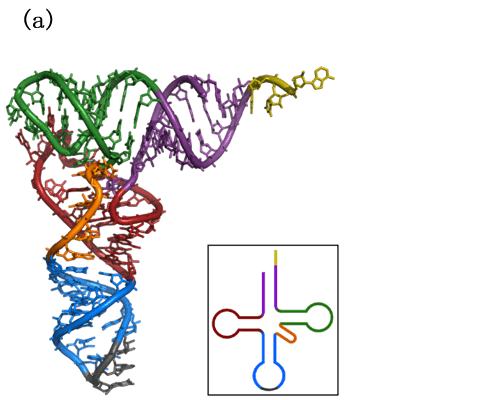

Figure.1 Non-coding RNA. (a) Molecular structure of alanine tRNA derived from yeast. (b) Complementation of the central dogma by noncoding RNAs.
What is MicroRNA (miRNA)?
MicroRNAs (miRNAs) are a kind of small single-stranded non-coding RNA containing about 22 nucleotides[11]. According to genomics analysis, the human genome encodes up to more than 1900 miRNAs, and in the latest bioinformatics-based analysis this number can be as high as 2300[12, 13]. However, after manual sorting, it is finally confirmed that the number of human miRNAs is only about 500, and miRNAs whose physiological functions have been identified are still few[14]. The first miRNA was identified in the 1990s[15]. And after the 2000s, it was identified as an important regulatory factor and gradually became a research hotspot[16]. MiRNAs are involved in many pathological processes of various diseases, including cancer, heart disease, nerve system diseases, kidney diseases, obesity, etc. And some of the miRNA play key roles in the basic life activities of human growth, development, and survival, especially some genetically conserved miRNAs[17].
Molecular mechanism and Research Methods of miRNA
Synthesis and Functions of miRNA
Precursor miRNA (pre-miRNA) is obtained by transcription, and it can form a self-complementary folded hairpin structure (Figure.2). This hairpin-like primary transcript is cleaved through the action of Dicer to form two miRNAs, a miRNA 5p (miRNA) near the 5′ end and a miRNA 3p (miRNA*) near the 3′ end. MiRNA 3p is usually rapidly degraded, and the remaining miRNA 5p is usually considered to be the mature form of miRNAs. Most miRNAs 3p are short-lived and useless, but it was found that some of the miRNAs 3p also have important physiological and pathological functions, identical to miRNAs generally considered mature [18]. MiRNA mainly exerts its function by blocking the expression of target genes by mediating the transcription and translation of target genes. MiRNAs can bind to mRNA by complementary base pairing. This leads to three possibilities, including the degradation of the mRNA, the shortening of the polyA tail of the mRNA, and the blocking of the translation process involving ribosomes, which ultimately lead to blocked translational processes of mRNAs. Besides, the matching sequence of animal miRNAs and the target genes is too short (at least 6-8bp), which makes animal miRNA have many targets and difficult for a single miRNA to block the expression of the target gene[19]. Therefore, combinatorial regulation of multiple miRNAs is a common way for animal miRNAs to function[20].

Figure.2 Structure of Pre-miRNA
Research Methods of miRNA
Quantification of miRNAs can usually be performed by RT-PCR-based methods, such as stem-loop RT-PCR and polyA RT-PCR[21, 22]. But due to the small size of miRNA molecules, the high-throughput analysis process has a high error rate. Therefore, it is a common method to determine the role of miRNA by the level of target gene mRNA[23]. In project design and experimental operation, miRNA mimics, inhibitors, agomir, and antagomir are widely used in animal and cell experiments for miRNA-related research. These additives can mimic miRNA function or as miRNA inhibitors are used in functional experiments. Mimics are a kind of chemically synthesized, double-stranded miRNA-like RNA, typically used for cell transfection[24]. And agomirs are a kind of chemically modified mimics of miRNA, it has stronger membrane binding capacity and is less prone to degradation, typically used in the animal models and enables non-transfected cell treatment[25].
Examples of miRNA-related publications
Combining in vivo experiments with animal models and in vitro experiments with cellular models is a routine strategy for miRNA research. In a publication on liver cancer, Feng, et al. revealed the role of miR-149 3p in hepatocellular carcinoma (HCC) through miRNA mimics-based cellular models and agomir-based mouse models[18]. In the in vivo experimental part, they establish HCC mouse models (LPS or DEN exposed) based on C57 WT and miR-149 3p KO mice and then treat the mice with miR-149 3p agomir. In the in vitro experimental part, they transfected miR-149 3p mimics, LPS, TNF-α, and p65 separately or jointly for liver cancer cell lines. Besides, a tumorigenic model was established by miR-149 3p mimics-treated HCC cell lines and BLAB-C nude mice. Combining the results of the above in vivo and in vitro experiments, they find that miR-149 3p can inhibit the progression of HCC by down-regulate the expression of TNF-α-TRADD-NF-κB signal-related death domain protein.
Conclusion
MiRNA is a very hot research field in the past 10 years, and the number of publications has been at a high level in recent years (Figure.3). For researchers who want to enter this field, it is necessary to master the strategies and methods related to miRNA research.

Figure.3 Number of miRNA-related publications in recent decades (form Pubmed).
Where to get MicroRNA products and services?
AcceGen has pioneered the development of microRNA research and diagnostics tools with leading-edge services. AcceGen supplies ready-to-use MicroRNA Agomir/Antagomir for your basic research, covering 3 species: Human, Mouse and Rat. We also provide MicroRNA Agomir and Antagomir synthesis and MicroRNA Sponge services. We’re committed to paving new ways to meet all your needs for miRNA -related researches.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact inquiry@accegen.com.
References
1. Non-coding RNAs and Epigenetic Regulation of Gene Expression: Drivers of Natural Selection. Caister Academic Press; 2012.
2. Perkel JM: Visiting “noncodarnia”. Biotechniques 2013, 54:301, 303-304.
3. Hüttenhofer A, Schattner P, Polacek N: Non-coding RNAs: hope or hype? Trends Genet 2005, 21:289-297.
4. Brosius J: Waste not, want not–transcript excess in multicellular eukaryotes. Trends Genet 2005, 21:287-288.
5. Holley RW, Apgar J, Everett GA, Madison JT, Marquisee M, Merrill SH, Penswick JR, Zamir A: STRUCTURE OF A RIBONUCLEIC ACID. Science 1965, 147:1462-1465.
6. Ladner JE, Jack A, Robertus JD, Brown RS, Rhodes D, Clark BF, Klug A: Structure of yeast phenylalanine transfer RNA at 2.5 A resolution. Proc Natl Acad Sci U S A 1975, 72:4414-4418.
7. Kim SH, Quigley GJ, Suddath FL, McPherson A, Sneden D, Kim JJ, Weinzierl J, Rich A: Three-dimensional structure of yeast phenylalanine transfer RNA: folding of the polynucleotide chain. Science 1973, 179:285-288.
8. Wang J, Zhu S, Meng N, He Y, Lu R, Yan GR: ncRNA-Encoded Peptides or Proteins and Cancer. Mol Ther 2019, 27:1718-1725.
9. Ziats MN, Rennert OM: Aberrant expression of long noncoding RNAs in autistic brain. J Mol Neurosci 2013, 49:589-593.
10. Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, Finch CE, St Laurent G, 3rd, Kenny PJ, Wahlestedt C: Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med 2008, 14:723-730.
11. Qureshi A, Thakur N, Monga I, Thakur A, Kumar M: VIRmiRNA: a comprehensive resource for experimentally validated viral miRNAs and their targets. Database (Oxford) 2014, 2014.
12. University M: Homo sapiens miRNAs in the miRBase.
13. Alles J, Fehlmann T, Fischer U, Backes C, Galata V, Minet M, Hart M, Abu-Halima M, Grässer FA, Lenhof HP, et al: An estimate of the total number of true human miRNAs. Nucleic Acids Res 2019, 47:3353-3364.
14. Fromm B, Domanska D, Høye E, Ovchinnikov V, Kang W, Aparicio-Puerta E, Johansen M, Flatmark K, Mathelier A, Hovig E, et al: MirGeneDB 2.0: the metazoan microRNA complement. Nucleic Acids Res 2020, 48:D132-d141.
15. Lee RC, Feinbaum RL, Ambros V: The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75:843-854.
16. Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G: The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 2000, 403:901-906.
17. Fromm B, Billipp T, Peck LE, Johansen M, Tarver JE, King BL, Newcomb JM, Sempere LF, Flatmark K, Hovig E, Peterson KJ: A Uniform System for the Annotation of Vertebrate microRNA Genes and the Evolution of the Human microRNAome. Annu Rev Genet 2015, 49:213-242.
18. Feng Q, Zhang H, Nie X, Li Y, Chen WD, Wang YD: miR-149* Suppresses Liver Cancer Progression by Down-Regulating Tumor Necrosis Factor Receptor 1-Associated Death Domain Protein Expression. Am J Pathol 2020, 190:469-483.
19. Bartel DP: MicroRNAs: target recognition and regulatory functions. Cell 2009, 136:215-233.
20. Rajewsky N: microRNA target predictions in animals. Nat Genet 2006, 38 Suppl:S8-13.
21. Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al: Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 2005, 33:e179.
22. Luo X, Zhang J, Wang H, Du Y, Yang L, Zheng F, Ma D: PolyA RT-PCR-based quantification of microRNA by using universal TaqMan probe. Biotechnol Lett 2012, 34:627-633.
23. Nielsen JA, Lau P, Maric D, Barker JL, Hudson LD: Integrating microRNA and mRNA expression profiles of neuronal progenitors to identify regulatory networks underlying the onset of cortical neurogenesis. BMC Neurosci 2009, 10:98.
24. Lu TX, Rothenberg ME: MicroRNA. J Allergy Clin Immunol 2018, 141:1202-1207.
25. abm: https://www.abmgood.com/Synthetic-miRNA-Mimics-and-Agomirs.html.
- Get link
- X
- Other Apps
Comments
Post a Comment